ПУБЛИКАЦИИ
КЛИНИЧЕСКИЕ ИССЛЕДОВАНИЯ
CYSTIC FIBROSIS (CF) is a hereditary disease caused by a mutation of the CFTR gene (Cystic Fibrosis Transmembrane conduction Regulator) and characterized by damage to the endocrine glands, as well as vital organs and systems, including severe respiratory disorders.
The most effective drug for targeted therapy of cystic fibrosis today is the CFTR modulator elecsacaftor / tezacaftor / ivacaftor. It is a combination of two CFTR correctors and a CFTR potentiator that correct the main genetic defect in patients with CF who carry at least one variant in the genotype specified in the instructions for the drug.
The most effective drug for targeted therapy of cystic fibrosis today is the CFTR modulator elecsacaftor / tezacaftor / ivacaftor. It is a combination of two CFTR correctors and a CFTR potentiator that correct the main genetic defect in patients with CF who carry at least one variant in the genotype specified in the instructions for the drug.
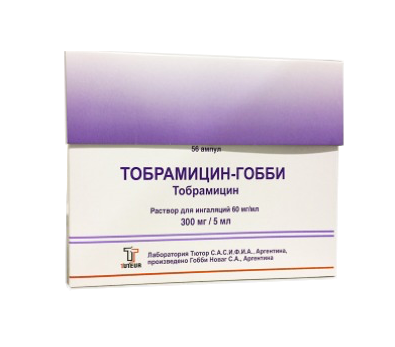
Tobramycin-Gobbi
Tobramycin, inhalation solution 60 mg/ml 5 ml
Tobramycin, inhalation solution 60 mg/ml 5 ml
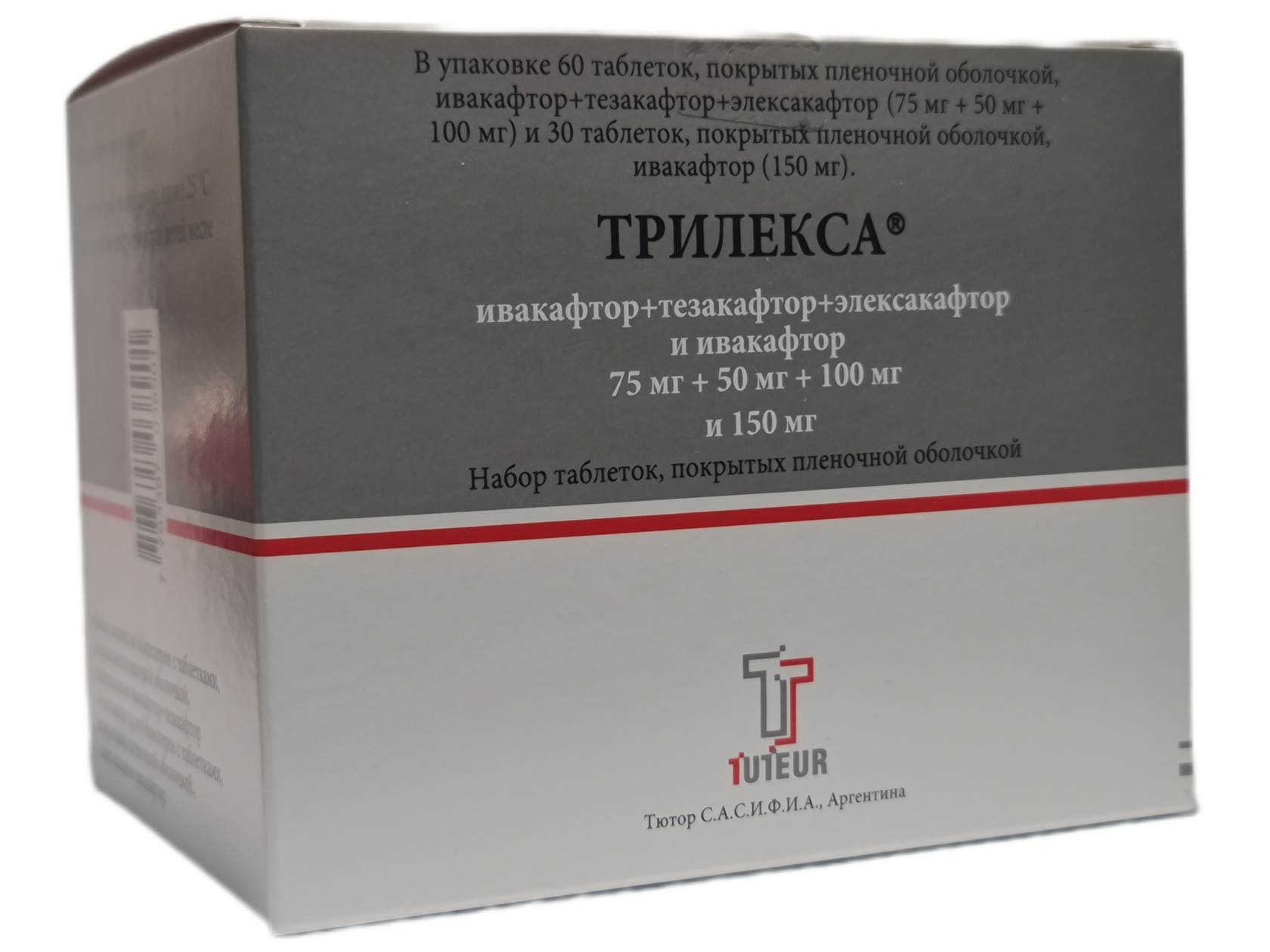
Trilexa® 37.5 mg + 25 mg + 50 mg; 75 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 37.50 mg of ivacaftor, 25.00 mg of tezacaftor and 50.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 75.00 mg of ivacaftor.
Trilexa® 75 mg + 50 mg + 100 mg; 150 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 75.00 mg of ivacaftor, 50.00 mg of tezacaftor and 100.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 150.00 mg of ivacaftor.
Each film-coated tablet, light blue in color, contains 75.00 mg of ivacaftor.
Trilexa® 75 mg + 50 mg + 100 mg; 150 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 75.00 mg of ivacaftor, 50.00 mg of tezacaftor and 100.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 150.00 mg of ivacaftor.
PULMONOLOGY
Experience of using the generic drug elecsacaftor / tezacaftor / ivacaftor + ivacaftor in patients with cystic fibrosis in routine clinical practice
S.A.Krasovsky1, 2, R.U.Kagazezhev1
1 Federal State Budgetary Institution "Scientific Research Institute of Pulmonology" of the Federal Medical and Biological Agency of Russia: 115682, Russia, Moscow, Orekhovy Boulevard, 28
2 Federal State Budgetary Scientific Institution "N.P.Bochkov Medical and Genetic Research Center" of the Ministry of Science and Higher Education of the Russian Federation: 1 Moskvorechye str., Moscow, 115522, Russia
The first experience of using targeted therapy in children with cystic fibrosis in Kazakhstan
Tatiana V. Marshalkina 1, https://orcid.org/0000-0001-6320-3241
Nazgul. Zhanuzakova 1, https://orcid.org/0000-0002-8474-4706
Irina Y. Mukatova 2, https://orcid.org/0000-0002-5804-8643
Svetlana S. Kim 3, https://orcid.org/0009-0008-5057-106X
Elena L. Amelina 2,4, https://orcid.org/0000-0002-5356-9415
1 JSC "Scientific Center of Pediatrics and Pediatric Surgery", Almaty, Republic of Kazakhstan; NAO
2 "Astana Medical University", Astana, Republic of Kazakhstan;
3 Educational Center "Viamedis Academy Limited", Astana, Republic of Kazakhstan;
4 Scientific Research Institute of Pulmonology of the Federal Medical and Biological
S.A.Krasovsky1, 2, R.U.Kagazezhev1
1 Federal State Budgetary Institution "Scientific Research Institute of Pulmonology" of the Federal Medical and Biological Agency of Russia: 115682, Russia, Moscow, Orekhovy Boulevard, 28
2 Federal State Budgetary Scientific Institution "N.P.Bochkov Medical and Genetic Research Center" of the Ministry of Science and Higher Education of the Russian Federation: 1 Moskvorechye str., Moscow, 115522, Russia
The first experience of using targeted therapy in children with cystic fibrosis in Kazakhstan
Tatiana V. Marshalkina 1, https://orcid.org/0000-0001-6320-3241
Nazgul. Zhanuzakova 1, https://orcid.org/0000-0002-8474-4706
Irina Y. Mukatova 2, https://orcid.org/0000-0002-5804-8643
Svetlana S. Kim 3, https://orcid.org/0009-0008-5057-106X
Elena L. Amelina 2,4, https://orcid.org/0000-0002-5356-9415
1 JSC "Scientific Center of Pediatrics and Pediatric Surgery", Almaty, Republic of Kazakhstan; NAO
2 "Astana Medical University", Astana, Republic of Kazakhstan;
3 Educational Center "Viamedis Academy Limited", Astana, Republic of Kazakhstan;
4 Scientific Research Institute of Pulmonology of the Federal Medical and Biological
The holder of the registration certificate for the drug "TRILEXA®" (Registration certificate number - LP-No.(007693)-(RG-RU)) is Tyutor S.A.S.I.F.I.A., Argentina.
Tyutor S.A.S.I.F.I.A. Company, Argentina (www.tuteur.com.ar) is a manufacturing company with more than 50 years of experience in the pharmaceutical industry and the promotion of high-tech drugs in such areas as oncology, hematology, nephrology, pulmonology, diseases of the central nervous system, autoimmune and orphan diseases, exports pharmaceutical products to all over the world since the 80s.
Tyutor has two own production facilities in Argentina with a total area of 7,500 m2 with an installed production capacity of more than 105 million metered units per year, which allows it to meet national and international demand for the company's medicines.
In addition to its own pharmaceutical production and export of pharmaceutical products to Europe, Latin America and the CIS/EAEU, Tyutor has also been exporting raw materials for the pharmaceutical industry to European countries - France, Germany and Italy since 1980.
Tyutor focuses on comprehensive quality management: adheres to strict rules of good manufacturing practice, uses strict standard operating procedures for incoming quality control of pharmaceutical substances and excipients. The company's production sites comply with the requirements of good manufacturing Practice (GMP) and have a valid GMP certificate issued by the National Institute of Medicines, the expert authority for Medicines, Food and Medical Technologies of Argentina (A.N.M.A.T. – national regulatory structure, equivalent to the FDA, USA). Compliance with the requirements of good manufacturing practice (GMP) in accordance with the current regulations of the Argentine Republic, as part of the application of the recommendations of the World Health Assembly (WHA) and the International Conference on Harmonization (ICH) is checked regularly as part of inspections for the annual renewal of the GMP certificate.
Since 2008, the Office of Medicines, Food and Medical Technologies of Argentina (A.N.M.A.T.) has been an active member of the international PIC/S (Pharmaceutical Inspection Co-operationScheme - Pharmaceutical Inspection Cooperation Scheme) scheme, which confirms the recognition by the global industry community of the level of pharmaceutical production in the country and its proper inspection and guarantees a consistently high the quality of the company's pharmaceutical products.
Tyutor's portfolio includes more than 120 products, 23 of which are registered and have been supplied to the pharmaceutical market of the Russian Federation for about 30 years. During this time, no complaints have been received regarding the quality of pharmaceutical products from this manufacturer.
Tyutor S.A.S.I.F.I.A. Company, Argentina (www.tuteur.com.ar) is a manufacturing company with more than 50 years of experience in the pharmaceutical industry and the promotion of high-tech drugs in such areas as oncology, hematology, nephrology, pulmonology, diseases of the central nervous system, autoimmune and orphan diseases, exports pharmaceutical products to all over the world since the 80s.
Tyutor has two own production facilities in Argentina with a total area of 7,500 m2 with an installed production capacity of more than 105 million metered units per year, which allows it to meet national and international demand for the company's medicines.
In addition to its own pharmaceutical production and export of pharmaceutical products to Europe, Latin America and the CIS/EAEU, Tyutor has also been exporting raw materials for the pharmaceutical industry to European countries - France, Germany and Italy since 1980.
Tyutor focuses on comprehensive quality management: adheres to strict rules of good manufacturing practice, uses strict standard operating procedures for incoming quality control of pharmaceutical substances and excipients. The company's production sites comply with the requirements of good manufacturing Practice (GMP) and have a valid GMP certificate issued by the National Institute of Medicines, the expert authority for Medicines, Food and Medical Technologies of Argentina (A.N.M.A.T. – national regulatory structure, equivalent to the FDA, USA). Compliance with the requirements of good manufacturing practice (GMP) in accordance with the current regulations of the Argentine Republic, as part of the application of the recommendations of the World Health Assembly (WHA) and the International Conference on Harmonization (ICH) is checked regularly as part of inspections for the annual renewal of the GMP certificate.
Since 2008, the Office of Medicines, Food and Medical Technologies of Argentina (A.N.M.A.T.) has been an active member of the international PIC/S (Pharmaceutical Inspection Co-operationScheme - Pharmaceutical Inspection Cooperation Scheme) scheme, which confirms the recognition by the global industry community of the level of pharmaceutical production in the country and its proper inspection and guarantees a consistently high the quality of the company's pharmaceutical products.
Tyutor's portfolio includes more than 120 products, 23 of which are registered and have been supplied to the pharmaceutical market of the Russian Federation for about 30 years. During this time, no complaints have been received regarding the quality of pharmaceutical products from this manufacturer.

Trilexa®
(elecsacaftor / tezacaftor / ivacaftor + ivacaftor)
Registration certificate number LP-No. (007693)-(RG-RU)
Orphan drug status
Recommended dose of Triplex®
for adult patients and children aged 6 years and older
GENERAL INFORMATION
BRIEF DESCRIPTION OF THE DRUG "Trilexa®"
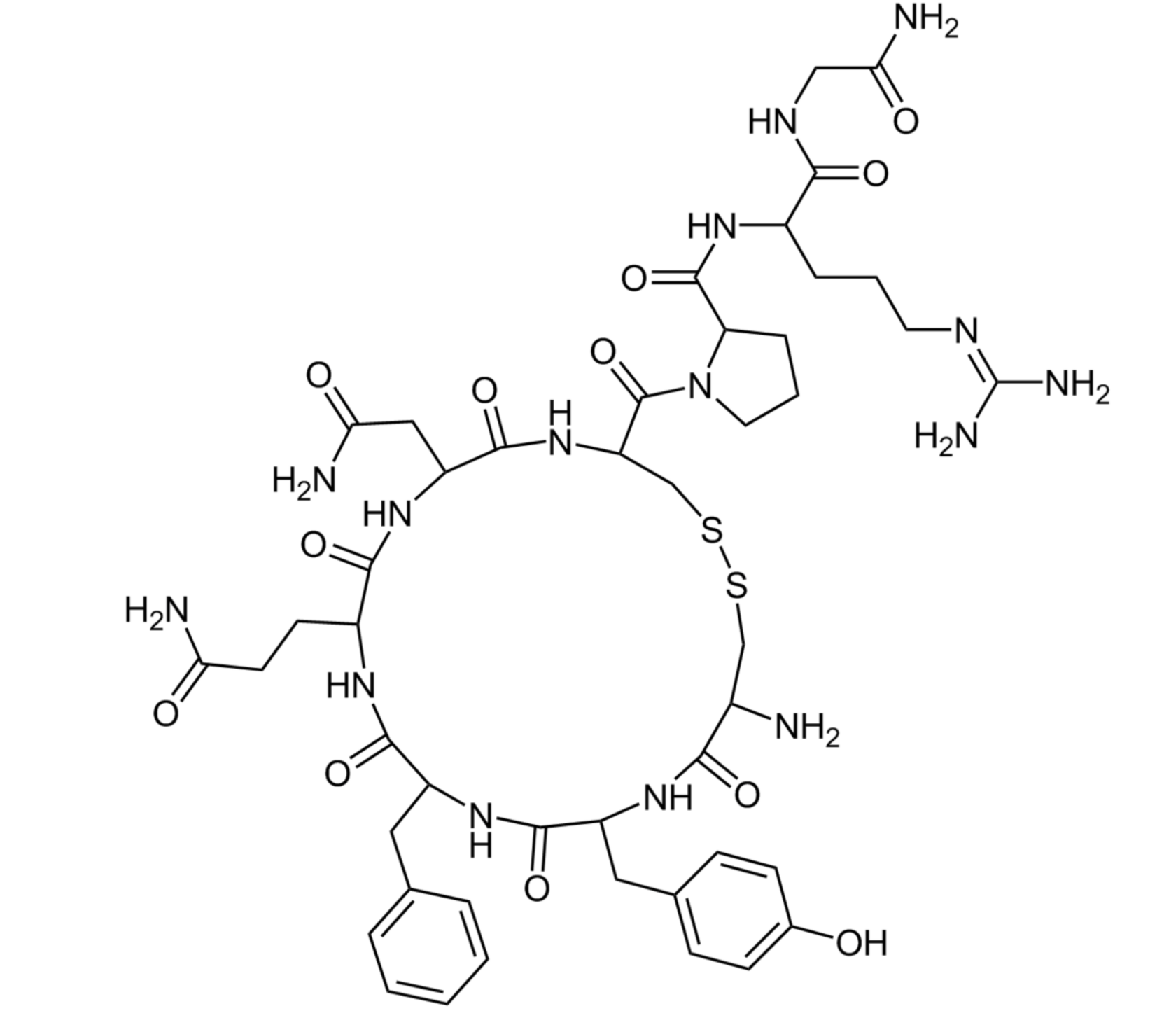
The drug "Trilexa®" is a combined medicinal product and is a fixed combination of doses of three active ingredients ivacaftor + tezacaftor + elecsacaftor and ivacaftor.
The drug "Trilexa®" belongs to the pharmacotherapeutic group "other drugs for the treatment of diseases of the respiratory system", ATC code: R07AX32.
The drug "Trilexa®" is registered in the country of manufacture, Argentina, as well as in the Russian Federation.
Additional signs of the drug
The indication for use with the ICD-10 code "E84" is included in the official list of rare (orphan) diseases.
The result of the examination within the framework of the registration procedure according to the requirements of the EAEU
The quality, efficacy and safety of the drug Trilex® have been confirmed according to the data of the Expert Report of the Federal State Budgetary Institution "NCESMP" of the Ministry of Health of the Russian Federation dated 11/14/2024 No. 25161.
Information about dosage forms and dosage
Trilexa® 37.5 mg + 25 mg + 50 mg; 75 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 37.50 mg of ivacaftor, 25.00 mg of tezacaftor and 50.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 75.00 mg of ivacaftor.
Trilexa® 75 mg + 50 mg + 100 mg; 150 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 75.00 mg of ivacaftor, 50.00 mg of tezacaftor and 100.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 150.00 mg of ivacaftor.
Brief description of the mechanism of action
The drug Trilexa® is a CFTR (Cystic Fibrosis Transmembrane conduction Regulator) protein modulator.
The combination of elecsacaftor, tezacaftor, and ivacaftor increases the amount and function of the cystic fibrosis transmembrane conduction regulator (CFTR) protein on the cell surface, which leads to increased CFTR activity.
Elecsacaftor and thesacaftor act as CFTR correctors, restoring F508del processing by binding to the CFTR protein to increase the availability of the CFTR protein on the cell surface. They work by changing the shape of the CFTR protein to position it on the cell surface. The combination of increased CFTR protein content in the correct position on the cell surface with potentiation of chloride channel opening by ivacaftor leads to increased chloride transport and dilution of mucus secretion [1, 2].
The triple fixed combination of ivacaftor+tezacaftor+elecsacaftor (ELX/TEZ/IVA) modulators is a new step in providing effective treatment for patients with cystic fibrosis. A triple combination of the ivacaftor potentiator (IVA) and two correctors elecsacaftor (ELX) and tezacaftor (TEZ) has become available for the treatment of more than 90% of people with CF who have at least one CFTR allele sensitive to this drug [3]. Due to a significant improvement in anthropometric data, lung function and quality of life, and a reduction in pulmonary exacerbations that have become achievable with triple therapy, the fixed combination of ELX/TEZ/IVA has been recognized as the standard treatment for CF at the present time [4]. Based on individual modeling of humans as a biological system using the method of microsimulation, the median life expectancy of homozygous patients with MB according to F508del was calculated in conditions of receiving targeted ELX/TEZ/IVA therapy and the best supportive therapy, which amounted to 71.6 years [5].
List of literature
1. Trikafta- elexacaftor, tezacaftor, and ivacaftor kit // Daily Med : [website]. – URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f354423a-85c2-41c3-a9db-0f3aee135d8d (date of access: 08.04.2022). – Text : electronic.
2. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy / A. Zaher, J. ElSaygh, D. Elsori [et al.]. – DOI: 10.7759/cureus.16144 // Cureus. – 2021. – Vol. 13, № 7. – P. e16144.
3. Peter G. Middleton, Marcus A. Mall, Pavel Dřevínek, Larry C. Lands, Edward F. McKone, Deepika Polineni, Bonnie W. Ramsey, et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med 2019;381:1809-19.
4. PScott C Bell, Marcus A Mall, Hector Gutierrez, Milan Macek, Susan Madge, Jane C Davies, et al. The future of cystic fibrosis care: a global perspective. The Lancet Respiratory Medicine Commission Volume 8, Issue 1 January 2020:65-124.
5. Andrea Lopez, Conor Dalyb, Gabriela Vega-Hernandezb, Gordon MacGregorc, Jaime L. Rubin. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. Journal of Cystic Fibrosis 22 (2023) 607–614. https://doi.org/10.1016/j.jcf.2023.02.004.
Indications for use
Trilexa® is indicated for the treatment of cystic fibrosis in patients aged 6 years and older with at least one F508del mutation in the cystic fibrosis transmembrane conduction regulator gene or a mutation in the CFTR gene that responds to the drug based on in vitro studies.
If the patient's genotype is unknown, a genetic test approved by a healthcare institution should be used to identify at least one F508del mutation or a mutation that responds to the drug based on in vitro studies.
Dosage regimen (brief information on the main target population)
The recommended dose for adult patients and children aged 6 years and older is shown in the table. The morning and evening dose should be taken approximately 12 hours apart.
Table. Recommended dose of Trilex® tablets for adult patients and children aged 6 years and older
BRIEF DESCRIPTION OF THE DRUG "Trilexa®"
The drug "Trilexa®" is a combined medicinal product and is a fixed combination of doses of three active ingredients ivacaftor + tezacaftor + elecsacaftor and ivacaftor.
The drug "Trilexa®" belongs to the pharmacotherapeutic group "other drugs for the treatment of diseases of the respiratory system", ATC code: R07AX32.
The drug "Trilexa®" is registered in the country of manufacture, Argentina, as well as in the Russian Federation.
Additional signs of the drug
The indication for use with the ICD-10 code "E84" is included in the official list of rare (orphan) diseases.
The result of the examination within the framework of the registration procedure according to the requirements of the EAEU
The quality, efficacy and safety of the drug Trilex® have been confirmed according to the data of the Expert Report of the Federal State Budgetary Institution "NCESMP" of the Ministry of Health of the Russian Federation dated 11/14/2024 No. 25161.
Information about dosage forms and dosage
Trilexa® 37.5 mg + 25 mg + 50 mg; 75 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 37.50 mg of ivacaftor, 25.00 mg of tezacaftor and 50.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 75.00 mg of ivacaftor.
Trilexa® 75 mg + 50 mg + 100 mg; 150 mg - a set of film-coated tablets. Each orange-pink film-coated tablet contains 75.00 mg of ivacaftor, 50.00 mg of tezacaftor and 100.00 mg of elecsacaftor.
Each film-coated tablet, light blue in color, contains 150.00 mg of ivacaftor.
Brief description of the mechanism of action
The drug Trilexa® is a CFTR (Cystic Fibrosis Transmembrane conduction Regulator) protein modulator.
The combination of elecsacaftor, tezacaftor, and ivacaftor increases the amount and function of the cystic fibrosis transmembrane conduction regulator (CFTR) protein on the cell surface, which leads to increased CFTR activity.
Elecsacaftor and thesacaftor act as CFTR correctors, restoring F508del processing by binding to the CFTR protein to increase the availability of the CFTR protein on the cell surface. They work by changing the shape of the CFTR protein to position it on the cell surface. The combination of increased CFTR protein content in the correct position on the cell surface with potentiation of chloride channel opening by ivacaftor leads to increased chloride transport and dilution of mucus secretion [1, 2].
The triple fixed combination of ivacaftor+tezacaftor+elecsacaftor (ELX/TEZ/IVA) modulators is a new step in providing effective treatment for patients with cystic fibrosis. A triple combination of the ivacaftor potentiator (IVA) and two correctors elecsacaftor (ELX) and tezacaftor (TEZ) has become available for the treatment of more than 90% of people with CF who have at least one CFTR allele sensitive to this drug [3]. Due to a significant improvement in anthropometric data, lung function and quality of life, and a reduction in pulmonary exacerbations that have become achievable with triple therapy, the fixed combination of ELX/TEZ/IVA has been recognized as the standard treatment for CF at the present time [4]. Based on individual modeling of humans as a biological system using the method of microsimulation, the median life expectancy of homozygous patients with MB according to F508del was calculated in conditions of receiving targeted ELX/TEZ/IVA therapy and the best supportive therapy, which amounted to 71.6 years [5].
List of literature
1. Trikafta- elexacaftor, tezacaftor, and ivacaftor kit // Daily Med : [website]. – URL: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f354423a-85c2-41c3-a9db-0f3aee135d8d (date of access: 08.04.2022). – Text : electronic.
2. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy / A. Zaher, J. ElSaygh, D. Elsori [et al.]. – DOI: 10.7759/cureus.16144 // Cureus. – 2021. – Vol. 13, № 7. – P. e16144.
3. Peter G. Middleton, Marcus A. Mall, Pavel Dřevínek, Larry C. Lands, Edward F. McKone, Deepika Polineni, Bonnie W. Ramsey, et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N Engl J Med 2019;381:1809-19.
4. PScott C Bell, Marcus A Mall, Hector Gutierrez, Milan Macek, Susan Madge, Jane C Davies, et al. The future of cystic fibrosis care: a global perspective. The Lancet Respiratory Medicine Commission Volume 8, Issue 1 January 2020:65-124.
5. Andrea Lopez, Conor Dalyb, Gabriela Vega-Hernandezb, Gordon MacGregorc, Jaime L. Rubin. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. Journal of Cystic Fibrosis 22 (2023) 607–614. https://doi.org/10.1016/j.jcf.2023.02.004.
Indications for use
Trilexa® is indicated for the treatment of cystic fibrosis in patients aged 6 years and older with at least one F508del mutation in the cystic fibrosis transmembrane conduction regulator gene or a mutation in the CFTR gene that responds to the drug based on in vitro studies.
If the patient's genotype is unknown, a genetic test approved by a healthcare institution should be used to identify at least one F508del mutation or a mutation that responds to the drug based on in vitro studies.
Dosage regimen (brief information on the main target population)
The recommended dose for adult patients and children aged 6 years and older is shown in the table. The morning and evening dose should be taken approximately 12 hours apart.
Table. Recommended dose of Trilex® tablets for adult patients and children aged 6 years and older


To study the efficacy, safety, and satisfaction assessment of inhaled Tobramycin-Gobbi therapy for Pseudomonas aeruginosa infection in children with cystic fibrosis
E.I.Kondratieva1, A.Y.Voronkova1, S.V.Trishina2, N.S.Snetkova2, T.I.Safonova3, E.B.Pavlinova4, M.M.Chepurna5, L.E.Kharakhashyan5, O.I.Golubtsova6, M.A.Skachkova7, Yu.A.Kondakova8, E.A.Enina9,10, E.V.Vodovozova10
1 Federal State Budgetary Scientific Institution "Medical and Genetic Research Center named after Academician N.P.Bochkov":
1 Moskvorechye str., Moscow, 115478, Russia
2 Medical Academy named after S.I.Georgievsky Federal State Autonomous Educational Institution of Higher Education
"V.I.Vernadsky Crimean Federal University" of the Ministry of Science and Higher Education of the Russian Federation: 295006, Russia,
Republic of Crimea, Simferopol, Lenin b., 5 / 7
3 Budgetary Healthcare Institution of the Omsk region "Regional Children's Clinical Hospital": 77 Kuibyshev St., Omsk, 644001, Russia
4 Federal State Budgetary Educational Institution of Higher Education Omsk State Medical University
Ministry of Health of the Russian Federation: 12 Lenin St., Omsk, 644099, Russia
5 State Budgetary Institution of the Rostov Region "Regional Children's Clinical Hospital": 33 1st Cavalry Army str., Rostov-on-Don, 344029, Russia
6 Budgetary institution of the Chuvash Republic "Republican Children's Clinical Hospital" of the Ministry of Health of the Chuvash Republic:
428020, Russia, Cheboksary, Fedora Gladkova str., 27
7 Federal State Budgetary Educational Institution of Higher Education "Orenburg State Medical University"
of the Ministry of Health of the Russian Federation: 460000, Russia, Orenburg, Sovetskaya St., M.Gorky St., lane. Dmitrievsky, 6
8 State Budgetary Healthcare Institution of the Novosibirsk Region "City Children's Clinical Hospital for Emergency Medical Care":
3 Krasny Prospekt, Novosibirsk, 630007, Russia
9 Stavropol Territory State Budgetary Healthcare Institution "Regional Children's Clinical Hospital": 3 Semashko str., Stavropol, 355029, Russia
10 Federal State Budgetary Educational Institution of Higher Education "Stavropol State Medical University"
of the Ministry of Health of the Russian Federation: 310 Mira St., Stavropol, 355017, Russia
E.I.Kondratieva1, A.Y.Voronkova1, S.V.Trishina2, N.S.Snetkova2, T.I.Safonova3, E.B.Pavlinova4, M.M.Chepurna5, L.E.Kharakhashyan5, O.I.Golubtsova6, M.A.Skachkova7, Yu.A.Kondakova8, E.A.Enina9,10, E.V.Vodovozova10
1 Federal State Budgetary Scientific Institution "Medical and Genetic Research Center named after Academician N.P.Bochkov":
1 Moskvorechye str., Moscow, 115478, Russia
2 Medical Academy named after S.I.Georgievsky Federal State Autonomous Educational Institution of Higher Education
"V.I.Vernadsky Crimean Federal University" of the Ministry of Science and Higher Education of the Russian Federation: 295006, Russia,
Republic of Crimea, Simferopol, Lenin b., 5 / 7
3 Budgetary Healthcare Institution of the Omsk region "Regional Children's Clinical Hospital": 77 Kuibyshev St., Omsk, 644001, Russia
4 Federal State Budgetary Educational Institution of Higher Education Omsk State Medical University
Ministry of Health of the Russian Federation: 12 Lenin St., Omsk, 644099, Russia
5 State Budgetary Institution of the Rostov Region "Regional Children's Clinical Hospital": 33 1st Cavalry Army str., Rostov-on-Don, 344029, Russia
6 Budgetary institution of the Chuvash Republic "Republican Children's Clinical Hospital" of the Ministry of Health of the Chuvash Republic:
428020, Russia, Cheboksary, Fedora Gladkova str., 27
7 Federal State Budgetary Educational Institution of Higher Education "Orenburg State Medical University"
of the Ministry of Health of the Russian Federation: 460000, Russia, Orenburg, Sovetskaya St., M.Gorky St., lane. Dmitrievsky, 6
8 State Budgetary Healthcare Institution of the Novosibirsk Region "City Children's Clinical Hospital for Emergency Medical Care":
3 Krasny Prospekt, Novosibirsk, 630007, Russia
9 Stavropol Territory State Budgetary Healthcare Institution "Regional Children's Clinical Hospital": 3 Semashko str., Stavropol, 355029, Russia
10 Federal State Budgetary Educational Institution of Higher Education "Stavropol State Medical University"
of the Ministry of Health of the Russian Federation: 310 Mira St., Stavropol, 355017, Russia

ПУБЛИКАЦИИ
КЛИНИЧЕСКИЕ ИССЛЕДОВАНИЯ
PHENYLKETONURIA is a hereditary disease of the fermentopathy group associated with impaired metabolism of amino acids, mainly phenylalanine.
Efkuria®
Sapropterin, 100 mg dispersible tablets No. 30
Sapropterin, 100 mg dispersible tablets No. 30
HEREDITARY FERMENTOPATHIES

ПУБЛИКАЦИИ
КЛИНИЧЕСКИЕ ИССЛЕДОВАНИЯ
Central diabetes insipidus is a serious disease of the hypothalamic–pituitary system, which is based on a defect in the synthesis or secretion of antidiuretic hormone (vasopressin).
Vasomyrin
5 ml (50 doses) nasal spray, 10 mcg/dose
5 ml (50 doses) nasal spray, 10 mcg/dose
ENDOCRINOLOGY

ПУБЛИКАЦИИ
КЛИНИЧЕСКИЕ ИССЛЕДОВАНИЯ
THERMAL BURN is damage to body tissues caused by the action of high temperature.
The second degree is damage to the keratinized epithelium to the germ layer.
The third degree is damage to all layers of the epidermis and dermis.
The second degree is damage to the keratinized epithelium to the germ layer.
The third degree is damage to all layers of the epidermis and dermis.
NexoBrid®
Active ingredient: bromelain 5.0 g complete with gel base
Lyophilizate for gel preparation for external use
Active ingredient: bromelain 5.0 g complete with gel base
Lyophilizate for gel preparation for external use
COMBUSTIOLOGY






